Creating smarter, simpler ethics and compliance processes
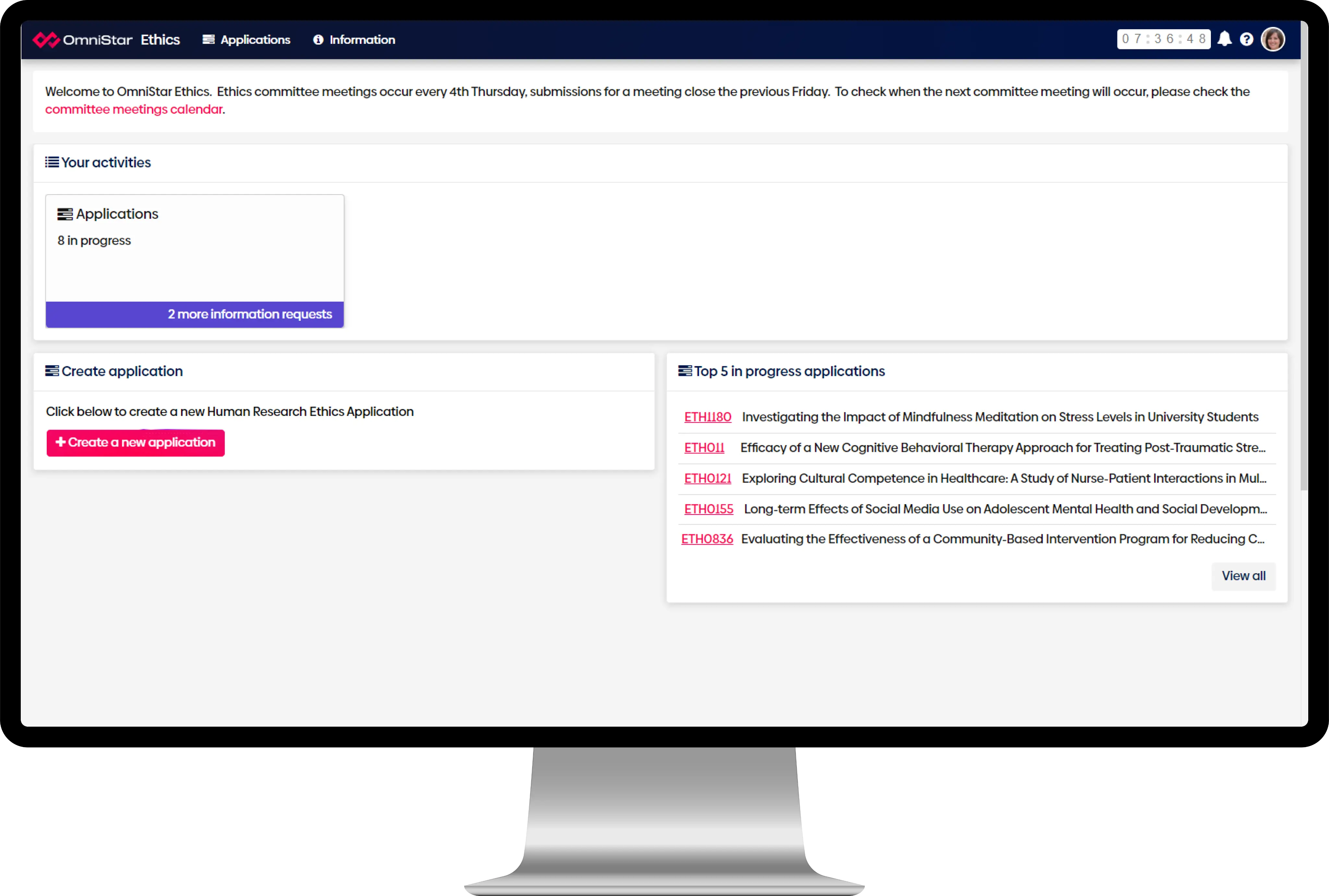
OmniStar Ethics gives you a structured way to manage every stage of ethics oversight. Our system supports human research, animal research, biosafety, gene technology and governance reviews. It also streamlines full compliance management for universities, medical research institutes and other organisations.
The aim is simple. Reduce risk. Improve consistency. Give your teams a clear and reliable system that keeps ethics and compliance work moving forward.
Trusted by industry leading organisations










Centralised reporting
Streamline and centralise reporting with a single, purpose-built ethics and compliance software solution.
Reduce administrative burden
Guided processes and automated workflows and alerts simplify tasks and save time.

Compliance and governance
Built-in legislative compliance means you’re always adhering to regulations and meeting ethical standards.

An all-in-one ethics and compliance solution
OmniStar supports any type of ethics oversight. It draws on the ‘NHMRC Research and Governance Handbook: For the National Approach’, which streamlines approval processes into a single ethical review.
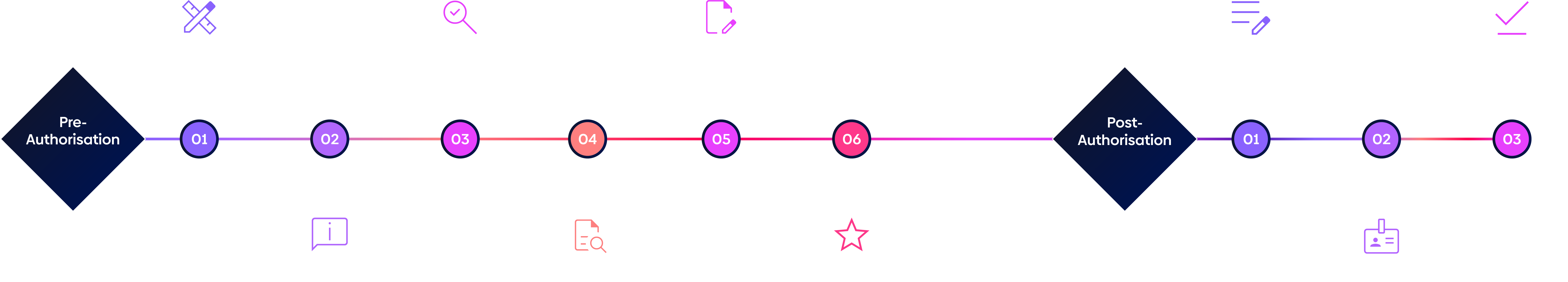
Pre and post approval
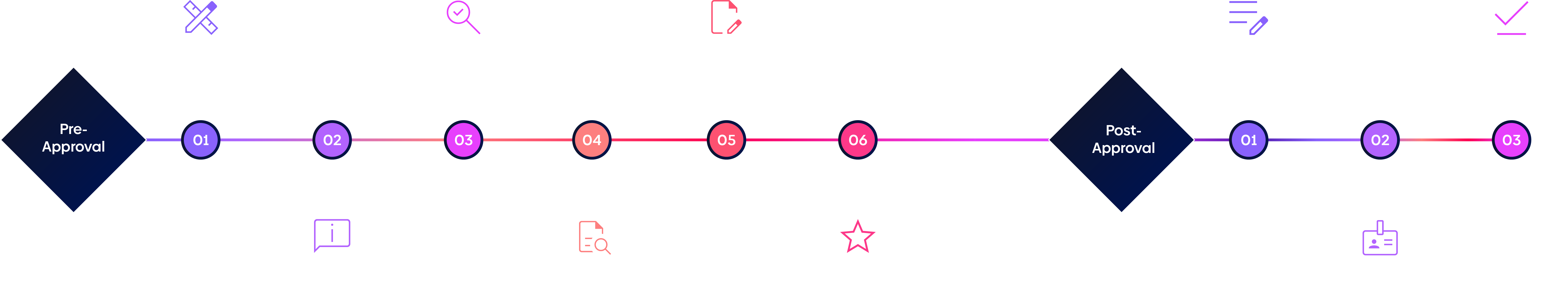
Pre and post authorisation
Save time
Data-driven design makes collating and presenting findings easier.
Report
Visualise trends and monitor progress with comprehensive, real-time reporting and analytics.
Comply
Compliance with regulations can be tracked and administered throughout the entire ethics approval process.
Our story
Ethics solutions for your industry
Features
OmniStar Ethics is a configurable, enterprise-grade ethics and compliance solution which streamlines approvals, integration and reporting.
Save time
Ethics processes
- Human, Animal ethics applications
- Compliance (e.g. Biosafety, gene technology)
- Governance - Site Specific Assessment
- HREA form (including export to ERM)
- Victorian Specific Module
- Low and negligible risk and high risk
- Integrated CRM
Applications and amendments
- Application form submission
- Invitation, sharing and collaboration
- Amendments including tracked changes
- Versioning (form and document)
- Copy and form pre-population
- Document versioning
Reviews and meetings
- Chat-like review commentary and applicant feedback
- Attendance recording, iCal invitations & online meeting
- Pre-meeting reviews and Conflict of Interest
- Request for more information
- Meeting agenda and minute auto-generation
- Single-file PDF meeting pack
Committees
- Automated committee allocation
- Committee membership & management including role management
- Recording of committee decisions
User-defined workflows & forms
- User-definable business rules
- Drag and drop workflow editor
- Customisable workflow steps
- Intuitive WYSIWYG SmartForm editor
- Pre-population, validation, branching logic and advanced functionality
- Configurable stages and statuses

Report
Progress reporting
- Adverse events/Safety reports
- Annual and final reports
- Report scheduling and automated reminders
- Report review and acceptance/approval
Dashboard & reporting
- Drag and drop dashboard and report editor
- Interactive (drill down/filter) dashboards and reports
- Pre-built dashboards and reports
- Dashboard & report sharing
Data analytics
- Data export (Excel, Image, PDF, etc)
- Temporal data warehouse
- Geospatial visualisations
- Enterprise Reporting Connector (ERC)
User-defined fields & pages
- User-definable fields, lookups and validation rules
- Configurable home page & tiles
- Customisable pages
- Configurable labels and text
User-defined templates
- Configurable document templates (Word, Excel, PDF, etc)
- Configurable email templates
- Tokenised email and document templates
- Branded email and document templates

Comply
Decisions and approvals
- Approval certificate generation including approved document list
- Conditional approval
- Digital decisions (full committee and executive level) & out-of-session approvals
- Approved animal numbers
- Facility management
Integration
- HR integration
- Document/records management integration
- Animal species integration
- ORCID integration
- Google+ integration
- Microsoft Teams integration
- Europe PMC integration
- DocuSign integration
- REST APIs & Webhooks
Security
- Configurable password policy
- Password blacklist validation
- Role & record-based security
- Single Sign-on
- Multi-factor Authentication (TOTP and Email)
- Advanced encryption (rest and in transit)
Certification & compliance
- IRAP and ISO 27001 certified
- GDPR & compliance with privacy principles
- Independent vulnerability & penetration testing
- Disaster recovery testing
- Load/performance testing
Ready to book a demo?
Integrations















Sunway University
OmniStar Ethics tailored the system to align with Sunway University’s unique needs, wants and budget.

Read our resources
Human research ethics application
In 2016, The National Health and Medical Research Council (NHMRC) of Australia, developed a new ethics application form which completely revolutionised the way in which research data is collected in human research tests.
OmniStar Ethics, the platform for REGIS
We are proud to announce that OmniEthics has been announced the platform for NSW Health’s Research Ethics and Governance Information System (REGIS).
Managing your ethics review panels
Managing ethics review panels can be a difficult & complex task. Add to that the need to manage the meetings, decisions and communication and the task can be complicated and time-consuming.
Problems that delay ethics approval and how to solve them
Discover the common reasons behind ethics approval delays, their impacts, and practical strategies to overcome them. Read more on our blog.
Ethics administration challenges and how to overcome them
Without effective ethics compliance solutions, institutions may face increased scrutiny and delays caused by administrative tasks. Using the right tools and technologies, organisations can streamline ethics management, ensuring both thoroughness and compliance.
How to streamline research ethics approval
By streamlining research ethics approval through intelligent workflows and automated coordination, institutions maintain robust ethical standards whilst eliminating unnecessary delays.
Strengthening research ethics: Why institutions need modern governance systems
Organisations implementing comprehensive ethics and compliance management software report faster approvals, improved committee efficiency, and greater stakeholder confidence.
Case studies
The University of Sydney (USYD)
USYD selected OmniStar Grants as their innovations platform to administer and manage their ethics program.
The University of Queensland
OmniStar has enabled the University of Queensland (UQ) to integrate their ethics management system with other UQ systems of record, reducing the time spent on data entry during the ethics application process and enabling data linkage across systems.
Sunway University
OmniStar Ethics tailored the system to align with Sunway University’s unique needs, wants and budget.
Frequently asked questions
Ethics compliance software is a type of software or SaaS program that helps organisations ensure that they are adhering to ethical standards and regulations. It typically includes tools for monitoring and managing compliance-related activities, such as policy development, training, incident reporting, and auditing. Ethics compliance software is important because it helps organisations avoid legal and reputational risks, promotes ethical behaviour among employees and stakeholders, and demonstrates their commitment to ethical standards.
Choosing the right ethics compliance software for your organisation depends on a variety of factors, such as your industry, size, budget, and specific compliance needs. Some key features to look for when evaluating ethics compliance software include automated policy management and distribution, customisable compliance workflows, integrated training and awareness tools, real-time monitoring and reporting capabilities, and the ability to integrate with other systems and data sources. It's also important to consider factors such as vendor reputation, customer support, and data security when selecting an ethics compliance software provider.
Ethics compliance software can help organisations manage risks related to data privacy and cybersecurity in several ways. For example, it can provide tools for tracking and reporting on data privacy compliance activities, such as data inventory and classification, data access and sharing controls, and incident response planning. It can also help organisations stay up to date with evolving cybersecurity threats and regulations, by providing automated risk assessments, vulnerability scans, and threat intelligence feeds. Additionally, many ethics compliance software providers offer built-in data security features, such as encryption, access controls, and secure data storage, to help organisations protect sensitive data and prevent unauthorised access or breaches.
All users rely on the system but each group will do so in their own way. Administrators might coordinate workflows or committee members conduct reviews, whilst researchers may submit and track any ongoing applications. Our compliance management solutions are built to provide support to every role.
Yes. The system supports animal research ethics, human research ethics, biosafety, and gene technology processes. The solution handles both animal research ethics and human research ethics in a single integrated system.
It uses SmartForms, validation rules, structured review stages, meeting tools, decision capture, digital approvals, and automated notifications.
Yes. Workflows, forms, rules, templates, and review steps can be set up and configured to fall in line with your organisation’s policies.
Utilise interactive dashboards, drill-down reports, scheduled reporting, data exports, and real-time analytics. All of this is available in one ethics compliance software solution.
Implementation is determined by process complexity and configuration needs. Most teams complete rollout through a structured onboarding plan that covers setup, testing, training, and launch.
Feature index
- Human, Animal ethics applications
- Compliance (e.g. Biosafety, gene technology)
- Governance - Site Specific Assessment
- HREA form (including export to ERM)
- Victorian Specific Module
- Low and negligible risk and high risk
- Integrated CRM
- Application form submission
- Invitation, sharing and collaboration
- Amendments including tracked changes
- Versioning (form and document)
- Copy and form pre-population
- Document versioning
- Chat-like review commentary and applicant feedback
- Attendance recording, iCal invitations & online meeting
- Pre-meeting reviews and Conflict of Interest
- Request for more information
- Meeting agenda and minute auto-generation
- Single-file PDF meeting pack
- Automated committee allocation
- Committee membership & management including role management
- Recording of committee decisions
- User-definable business rules
- Drag and drop workflow editor
- Customisable workflow steps
- Intuitive WYSIWYG SmartForm editor
- Pre-population, validation, branching logic and advanced functionality
- Configurable stages and statuses
- Adverse events/Safety reports
- Annual and final reports
- Report scheduling and automated reminders
- Report review and acceptance/approval
- Drag and drop dashboard and report editor
- Interactive (drill down/filter) dashboards and reports
- Pre-built dashboards and reports
- Dashboard & report sharing
- Data export (Excel, Image, PDF, etc)
- Temporal data warehouse
- Geospatial visualisations
- Enterprise Reporting Connector (ERC)
- User-definable fields, lookups and validation rules
- Configurable home page & tiles
- Customisable pages
- Configurable labels and text
- Configurable document templates (Word, Excel, PDF, etc)
- Configurable email templates
- Tokenised email and document templates
- Branded email and document templates
- Approval certificate generation including approved document list
- Conditional approval
- Digital decisions (full committee and executive level) & out-of-session approvals
- Approved animal numbers
- Facility management
- HR integration
- Document/records management integration
- Animal species integration
- ORCID integration
- Google+ integration
- Microsoft Teams integration
- DocuSign integration
- REST APIs & Webhooks
- Configurable password policy
- Password blacklist validation
- Role & record-based security
- Single Sign-on
- Multi-factor Authentication (TOTP and Email)
- Advanced encryption (rest and in transit)
- IRAP and ISO 27001 certified
- GDPR & compliance with privacy principles
- Independent vulnerability & penetration testing
- Disaster recovery testing
- Load/performance testing
Try out our ethics and compliance management software
Book a demo of OmniStar today and discover an effective, scalable and comprehensive ethics management software that maintains compliance and streamlines workflows.